Indication
To induce the production of antibodies to Chicken Anaemia Virus (CAV) by breeder hens and transfer of passive immunity to their progeny.
Presentation
1000 doses live freeze dried Chicken Anemia Virus strain Cux-1 per vial.
Vaccination Program
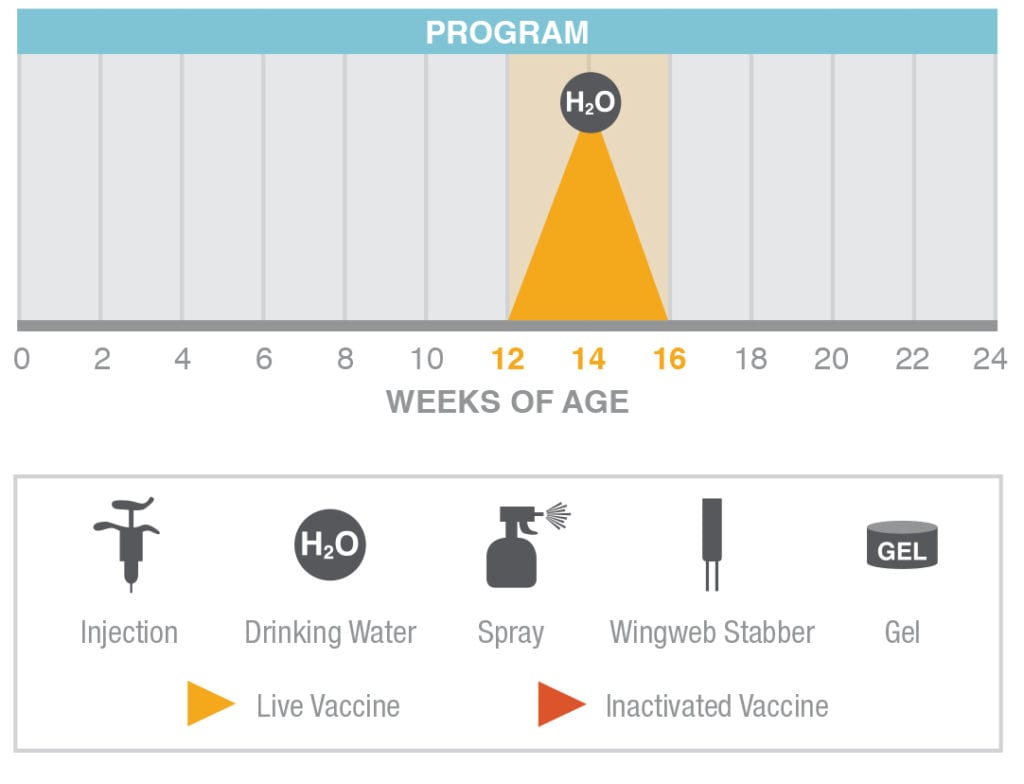
Layer Breeders and Broiler Breeders receive AviPro Thymovac® vaccine once during rearing.
Vaccine administration
From 12 until 16 weeks-of-age, but at least 6 weeks prior to the onset of lay administer AviPro Thymovac® in drinking water.
AviPro-Thymovac Program
Precautions
Store refrigerated at 2 to 7ºC. Do not allow to freeze.
Do not expose to disinfectants or heat.
The vaccine virus may spread from vaccinated birds.
Do not administer live CAV vaccine to breeders within 6 weeks of onset of lay. The CAV virus may be transmitted vertically in eggs following vaccination.
Birds less than 3 weeks-of-age infected with live CAV vaccine may display clinical disease, if they lack protection from passive transfer of maternal antibody.
See AviPro Thymovac® package insert for detailed Directions for Use.
AviPro Thymovac® registered pursuant to the ACVM Act 1997, No. A9132.See www.foodsafety.govt.nz for registration conditions.
Further Information
Disease Information Sheet
Chicken Anaemia Disease (CAV)
Administration Information Sheet
Drinking water administration of vaccine
Safety Card
AviPro Thymovac Safety Card
Product Information Sheet
AviPro Thymovac


